Human sperm and egg cell precursors made in lab for first time
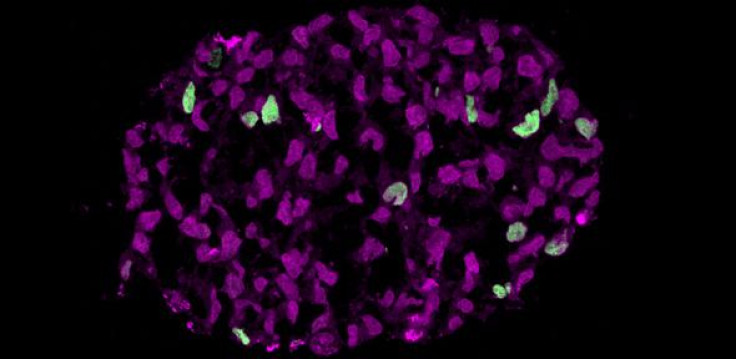
In a first, researchers from the Weizmann Institute of Science and Cambridge University have created in the lab primordial germ cells that give rise to sperm and ova.
This is the first time that human cells have been programmed into the early developmental stage, setting the stage for development of new reproductive technology to address fertility problems.
The work raises the possibility of enabling women who have undergone chemotherapy or premature menopause to conceive.
The research also has potential implications for understanding the process of "epigenetic" inheritance when changes to the genes caused by the environment are passed down generations. This process was shown to be erased during the process when the germ cells become specified.
"This could tell us how to erase these epigenetic mutations. Epigenetics is used to regulate gene expression, but in age-related diseases, these changes can be aberrant and misregulate genes," said Azim Surani, who led the work at the Gurdon Institute in Cambridge.
While human embryonic stem cells were used to form the germ cells, the group showed that it could be made from reprogrammed adult cells, such as skin cells, which will allow understanding better human germline, infertility and germ cell tumours.
The results of their study have been published in Cell.
"Researchers have been attempting to create human primordial germ cells (PGCs) in the petri dish for years," says Dr Jacob Hanna of the Weizmann Institute's Department of Molecular Genetics.
Process of programming
PGCs arise within the early weeks of embryonic growth when stem cells in the fertilised egg begin to differentiate into the many basic cell types. Once they become "specified" they progress into the sperm or ova cells.
Back in 2006 adult cells, or induced pluripotent stem (iPS) cells, were reprogrammed to act like embryonic cells that can then differentiate into any cell. In mice, these iPS cells were made to turn into germ cells.
The present work is the first attempt using human cells. The problem with human iPS cells was that despite the reprogramming to take them back to embryonic cell stage, they retained some memory of their origins.
Working further on them the team managed to turn them into "naïve cells" that could differentiate into any cell type, and possibly be coaxed to differentiate into primordial germ cells.
The scientists found that quite a high rate of the iPS cells – up to 40% – had become PGCs.
Challenges ahead
The steps from PGC to sperm and ova are complicated as the cells have to learn the neat trick of dividing their DNA in half.
In tracing the genetic chain of events that directs a stem cell to differentiate into a primordial germ cell, they discovered a master gene, Sox17, that regulates the process in humans, but not in mice.
Many questions remain before the cell programming can be translated into medical use, warns Hanna.
"For example, we found that only 'fresh' naïve cells can become PGCs; but after a week in conventional growth conditions they lose this capability once again. We want to know why this is. What is it about human stem cell states that make them more or less competent? And what exactly drives the process of differentiation once a cell has been reprogrammed to its more naïve state?"
© Copyright IBTimes 2024. All rights reserved.





