Mouse embryo artificially created from stem cells sheds light on early development
Scientists have successfully created an embryonic structure which resembles a mouse embryo.
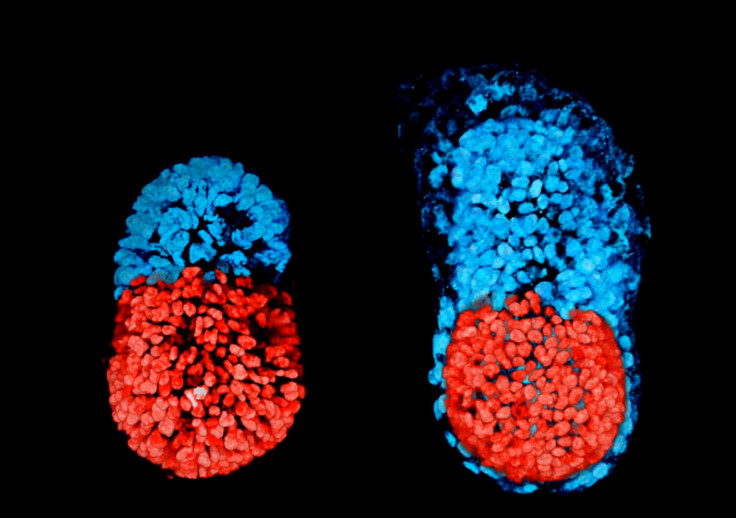
Scientists have successfully used stem cells to create artificially a structure resembling a mouse embryo. This achievement may help shine new light on a stage of early embryonic development, around the time of implantation in the womb, which remains poorly understood to date.
In mammalian reproduction, the egg is fertilised by the sperm, and then divides multiple times. After about five days, the embryo consists of three types of tissue, including an inner cell mass of embryonic stem cells (ESCs) that eventually go on to form the body.
The second type of cells, known as "trophoblast stem cells" (TSCs) differentiates to form the placenta, while the third type, known as primitive endoderm stem cells, ends up forming the yolk sac, which provides essential nourishment for the foetus and ensures that it develops properly.
Scientists have in the past attempted to create embryos artificially in the lab, without much success. However, they had only been using ESCs.
The authors of the new study, led by Professor Magdalena Zernicka-Goetz from the University of Cambridge, decided to try the experiment, hypothesising that embryo development requires different types of cells to work together and communicate.
"We've had the ability to generate embryonic stem cells in vitro and maintain them for about 40 years. But something these cells don't do very well by themselves is make a body. So our question was whether providing other cells to our model would recapitulate embryo architecture," Sarah Harrison, co-first author of the new study published in Science told IBTimes UK.
So here, the scientists used both mouse ESCs and TSCs, successfully forming an embryonic structure whose development and architecture was very similar to that of a normal, natural mouse embryo. It had anatomically correct regions, developing at the right place and the right time.
The research, now published in Science, thus suggests that distinct stem cells have an ability to assemble in-vitro and to coordinate, in order to generate embryo-like structures.
Not a healthy embryo
It is however unlikely that this artificial embryonic structure would develop into a normally-functioning, healthy foetus.

The main reason for this is that the model created by the team lacks the third type of stem cells found in natural embryos, and which goes on to form the yolk sac. "This third tissue that we don't represent in the model would be critical for onward development", Harrison said.
So what would this "artificial embryo" be useful for?
There are still a lot of questions relating to early embryonic development, particularly when it comes to understanding what goes on around the time of implantation in the mother's womb. It's that time of early development that the scientists have tried to represent in their model. In humans, it corresponds to a critical time, when two thirds of pregnancies fail.
The mouse embryo-like structure created by the scientists could provide an interesting model for embryologists to manipulate easily, and to study what goes at implantation. But it is uncertain how far the findings would be applicable to humans.
Nevertheless, research on the subject of early development is moving fast. Zernicka-Goetz recently published another major study describing a technique that allows embryos to develop in vitro beyond the implantation stage.
Now, the team is optimistic that they will one day be able to mimic a lot of the developmental events occurring early on, using human stem cells, in a similar way to what they have just done using mouse stem cells.
"The model as it stands is of a mouse embryo, not a human embryo, so the extent to which we can extrapolate to human pregnancy, there are still some questions marks over that. Although it would never be like the real thing, if we managed to create a similar model for human embryos, it would be useful to study disease progression and how it occurs, and perhaps screen for defects," Harrison pointed out.
Prof Simon Fishel Founder & President of Care Fertility Group told IBTimes UK:
"This study is about defining tissue development and interaction during the early post implantation period in the mouse. It is important work, using modelling and genetic signalling to evaluate which early cell lineages develop on into the embryonic tissues of the body and the important extra-embryonic tissues of the placenta..."
"However, as we know the human is different from the mouse it underscores the requirement to undertake similar studies in human embryos if we wish to understand how this manifests in our own species".
© Copyright IBTimes 2024. All rights reserved.






