Scientists create the rarest and most valuable material on the planet – metallic hydrogen
Material could be used as a superconductor and a powerful rocket propellant far beyond current fuels.
The "Holy Grail of high pressure physics" has been created by scientists – a material called atomic metallic hydrogen. The material, first theorized almost a century ago, is the rarest on the planet and could become one of the world's most valuable, having a wide applications – including as a powerful rocket propellant that could help us explore the far reaches of space.
Metallic hydrogen is a phase of hydrogen in which it acts as an electrical conductor. It was first theorized by physicists Eugene Wigner and Hillard Bell Huntington in 1935. They believed that under immense pressure, hydrogen atoms would display metallic properties, losing grip on their electrons.
Scientists at Harvard University have now said they have created these conditions – and metallic hydrogen – in the laboratory. Publishing their results in the journal Science, the team squeezed a hydrogen sample at at 495 gigapascal (GPa) – around 71.7 million pounds per square inch. To put that into perspective, that is more pressure than the centre of the Earth.
At this point, the solid molecular hydrogen breaks down and transform into atomic hydrogen – a metal. "This is the Holy Grail of high-pressure physics," said study author Isaac Silvera. "It's the first-ever sample of metallic hydrogen on Earth, so when you're looking at it, you're looking at something that's never existed before."
Importantly, researchers had to understand if metallic hydrogen is stable: "That means if you take the pressure off, it will stay metallic, similar to the way diamonds form from graphite under intense heat and pressure, but remain diamonds when that pressure and heat are removed," Silvera explained.
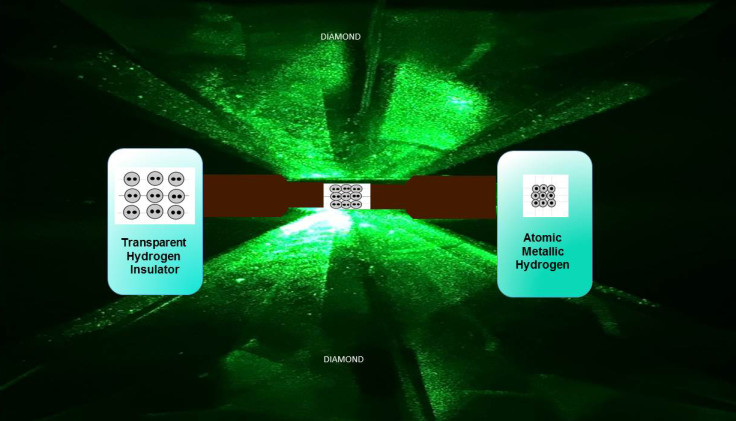
To create the material, the researchers used two synthetic diamonds tougher than those found in nature. They mounted them opposite each other and used them like an anvil, to squeeze the hydrogen together. The result – metallic hydrogen. "I immediately said we have to make the measurements to confirm it, so we rearranged the lab ... and that's what we did," Silvera said.
The applications for metallic hydrogen are huge. It has potential to be used as a room-temperature superconductor. Wires made from it could be used in the electrical grid – with normal conductors up to 15% of energy is lost in transmission, but this is not the case with atomic metal. As a result, it could be used to create high speed trains and better, more efficient electric cars.
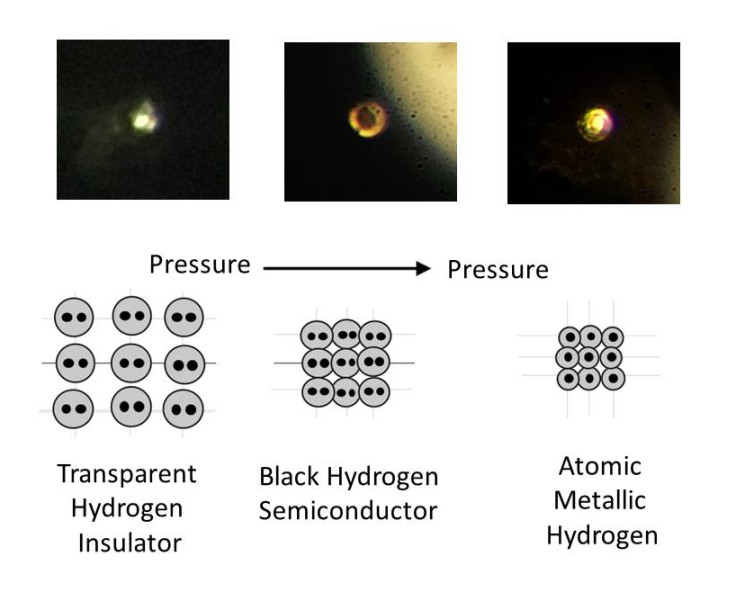
Another tantalising applications is as a rocket propellant. The "specific impulse" – a measure of how efficient rocket and jet engines are – is over treble that of our current best fuels. Silvera said: "It takes a tremendous amount of energy to make metallic hydrogen. And if you convert it back to molecular hydrogen, all that energy is released, so that would make it the most powerful rocket propellant known to man, and could revolutionise rocketry.
"That would easily allow you to explore the outer planets. We would be able to put rockets into orbit with only one stage, versus two, and could send up larger payloads, so it could be very important."
© Copyright IBTimes 2024. All rights reserved.






