World Malaria Day 2014: How Close Are We to a Vaccine?
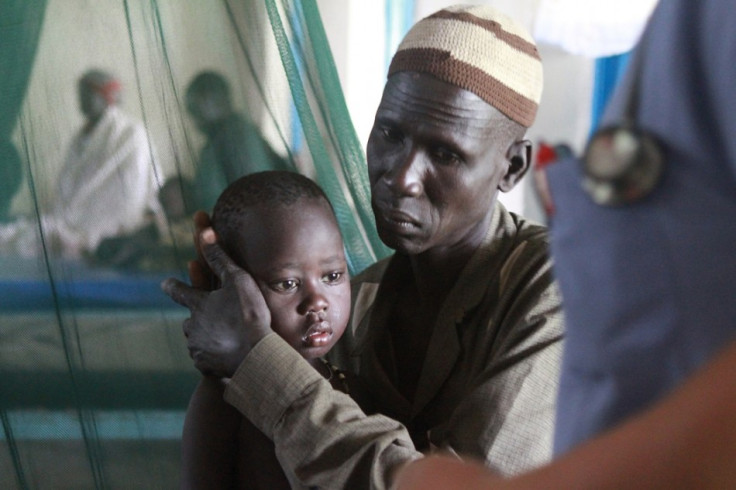
World Malaria Day 2014 is being held by the World Health Organisation today, focusing on investment in the future to defeat the most deadly disease on the planet.
Since 2000, WHO estimates about 3.3 million lives have been saved through global efforts to control and eliminate the disease.
Malaria deaths have fallen by 42% globally and 49% in Africa since 2000 – yet the disease is still responsible for the deaths of 627,000 people every year, mainly children under the age of five.
As well as these deaths, there are an estimated 200 million cases every year, with most never being tested or registered, and the development of an effective vaccine is essential to curb deaths from malaria on a wide scale.
Why isn't there a vaccine?
The complex nature of the malaria parasite means the development of a vaccine is extremely difficult. Despite several decades of research and development, as of yet there is no commercially available malaria vaccine. At present, there are 20 potential vaccines being tested in clinical trials or advanced preclinical development.
What is the best vaccine candidate?
At the moment, the most advanced candidate for a malaria vaccine is called RTS,S or Mosquirix.
A Phase III clinical trial for the vaccine has been completed with over 15,000 children from seven countries in sub-Saharan Africa participating.
The trial results showed the vaccine halved the number of malaria cases in children who received the vaccine aged between five and 17 months. It is designed to stop the malaria parasite from infecting, maturing and multiplying in the liver and infecting red blood cells.
Trial data needed for WHO to consider making a policy recommendation should be available at the end of 2014, meaning the vaccine could be rolled out as early as 2015.
How much further ahead is RTS,S than other candidates?
The clinical testing of RTS,S is at least five to ten years ahead of any other potential malaria vaccine currently in development. However, it is a vaccine against Plasmodium falciparum (one of the species that causes malaria), and does not protect against P. vivax malaria, THE most frequent and widely distributed cause of recurring malaria.
If RTS,S is rolled out, will other potential vaccines stop being funded?
No. Even if this potential malaria vaccine becomes WHO policy, further research and development into better and more effective vaccines will continue: "The potential role of RTS,S/AS01 will be in addition to fully scaled-up access to and use of nonvaccine malaria preventive measures, prompt diagnostic testing and effective anti-malarial medicines," WHO said.
"The need for high quality, safe and effective drugs to treat malaria will continue regardless of any deployment of a first-generation malaria vaccine such as RTS,S/AS01."
How much will it cost?
The vaccine was funded with a $200m (£147m) grant from the Bill & Melinda Gates Foundation and was developed by drugs giant GlaxoSmithKline. GSK has said it plans to sell the vaccine at cost price plus 5% - money that would be used to fund more research.
However, the realistic price of rolling out RTS,S is unknown and will have to be evaluated before it is used widely. WHO said: "The role of WHO, as the United Nations health agency, is to fully assess its safety and effectiveness; WHO will recommend RTS,S/AS01 if and when all required conditions for such a recommendation have been met.
"The introduction of a new vaccine is a major public health and financial decision that needs to be thoroughly assessed."
© Copyright IBTimes 2024. All rights reserved.






