Scientists harness power of photosynthesis to convert carbon dioxide into renewable fuels
New technique could help up us move away from reliance on greenhouse gas-emitting fossil fuels.
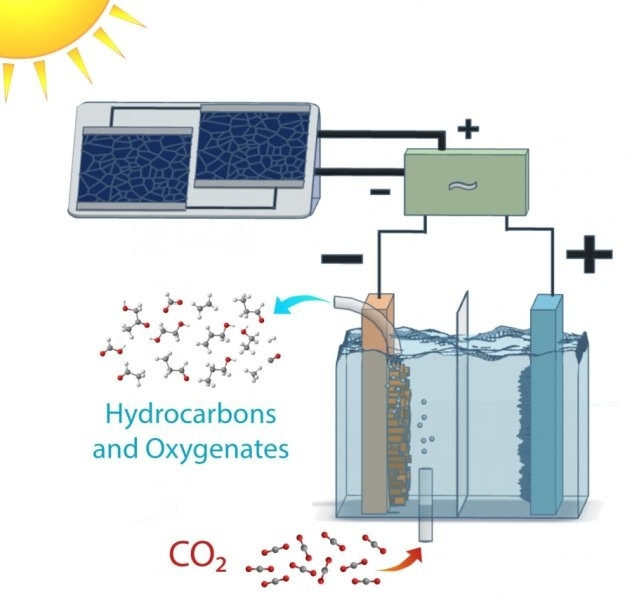
Scientists have developed an artificial technique that mimics natural photosynthesis to convert carbon dioxide into renewable fuels in a process that is more efficient than the equivalent process in plants.
The technology marks a significant milestone in efforts to move towards more sustainable sources of fuel.
Techniques have been developed before to produce fuel precursors from carbon dioxide. However, this study, published in the journal Energy and Environmental Science, is the first to show a technique that converts CO<sub>2 directly into its target products – in this case ethanol and ethylene – using sunlight at efficiencies rivalling their natural counterparts.
"This is an exciting development," said Joel Ager, lead author of the study. "As rising atmospheric CO<sub>2 levels change Earth's climate, the need to develop sustainable sources of power has become increasingly urgent. Our work here shows that we have a plausible path to making fuels directly from sunlight."
The study was conducted at the University of California Berkeley Campus of the Joint Center for Artificial Photosynthesis (JCAP) – an organisation set up to investigate technology that produces fuel directly from sunlight.
The system the team created achieved efficiency conversion rates of up to 5% – a difficult feat to achieve – and, furthermore, it was able to operate at all times of day, not just during peak sunlight hours.
"We did a little dance in the lab when we reached 5%", said Ager from Berkeley's Materials Science and Engineering Department.
Carbon dioxide is a stubbornly stable molecule and so breaking it apart to produce other molecules usually requires a significant input of energy.
"Reducing CO<sub>2 to a hydrocarbon end product like ethanol or ethylene can take up to 5 volts, start to finish," said Gurudayal Gurudayal, another lead author of the study. "Our system reduced that by half while maintaining the selectivity of products."
"By working through each step so carefully, these researchers demonstrated a level of performance and efficiency that people did not think was possible at this point," said Frances Houle, JCAP deputy director for Science and Research Integration, who was not part of the study.
Many researchers and policymakers have touted renewable fuels, such as ethanol, as possible alternatives to fossil fuels to mitigate the effects of global warming, at least in the short term, and in certain sectors, such as transport.
However, there is much debate over their effectiveness in achieving this. While some argue ethanol-based fuels are cleaner and release less greenhouse gases than petroleum fuels, others point to the fact that they can still contribute to other types of air pollution when burnt.
© Copyright IBTimes 2025. All rights reserved.





















