Harvesting the Sun: Scientists Turn Solar Power into Hydrogen Fuel Using Perovskite
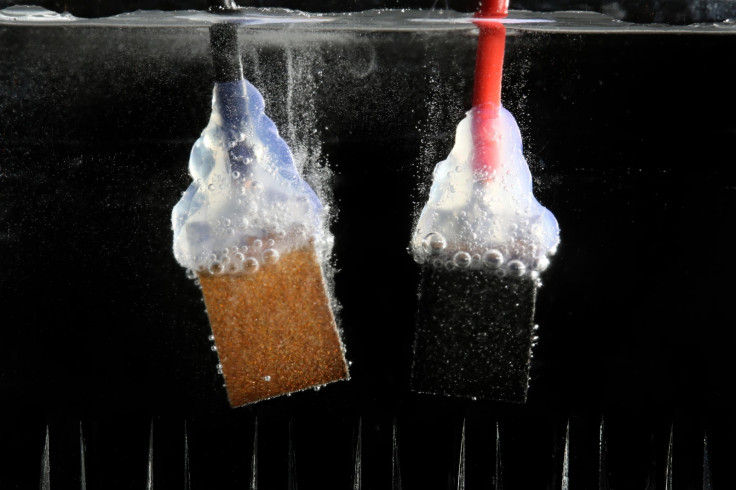
A group of Swiss scientists have succeeded in creating a device that can convert solar energy into hydrogen fuel at a much lower cost than ever before and which could help to harness solar power and solve the global energy crisis.
Researchers from the École Polytechnique Fédérale de Lausanne (EPFL), a Swiss federal institute of technology, split water that had been exposed to sunlight into separate hydrogen and oxygen cells using a solar cell device made from perovskite and a low-cost catalyst made from nickel and iron.
Their research, Water Photolysis At 12.3% Efficiency Via Perovskite Photovoltaics And Earth-Abundant Catalysts, is published in the latest issue of the journal Science.
Converting energy from water
In order to produce electrical energy from water, the process requires both a catalyst able to lower the activation energy barrier of splitting water so the reaction can occur and a solar cell - an electronic device converting the energy of light into electricity via the photovoltaic effect.
Solar cells are conventionally made from silicon but they are expensive to produce and require several cells to be used for each solar water splitting reaction, as silicon is not an extremely efficient absorber of light.
Although solar cells made from perovskite don't last for longer than a few hours, the cells were able to absorb 12.3% of the energy diffused by the sun and the researchers discovered using nickel and iron catalysts is key to improving the result from the reaction.
"Once you have hydrogen, you store it in a bottle and you can do with it whatever you want to, whenever you want it," said Dr Michael Grätzel, director of the Laboratory of Photonics and Interfaces, who led the research.
The hydrogen fuel gained from the process can be stored and used when needed to generate electricity, making the device a frontrunner in the global race to find a way to harness solar energy, as the technology used is easily affordable.
Higher voltage and cheaper
Grätzel has been working on solar cell research for many years and is famous for pioneering a new type of solar cell based on dye-sensitised nanocrystalline oxide films, known as the Grätzel cell.
He said the 12.3% solar cell conversion efficiency is only the beginning of what can be achieved as perovskite cells are able to generate a much higher open circuit voltage than silicon cells.
While silicon cells generate a maximum voltage of 0.7 V, each perovskite cell can produce a voltage greater than 1 V.
"A voltage of 1.7 V or more is required for water electrolysis to occur and to obtain exploitable gases," said Jingshan Luo, a post-doctorate scientist at EPFL's Laboratory of Photonics and Interfaces.
"This is the first time we have been able to get hydrogen through electrolysis with only two cells."
Meanwhile, the researchers' device is cheap to produce and doesn't need to use rare metals.
"Both the perovskite used in the cells and the nickel and iron catalysts making up the electrodes require resources that are abundant on Earth and that are also cheap," Luo said.
"However, our electrodes work just as well as the expensive platinum-based models customarily used."
© Copyright IBTimes 2025. All rights reserved.