Sperm have harpoon-like abilities to attach to egg, scientists believe
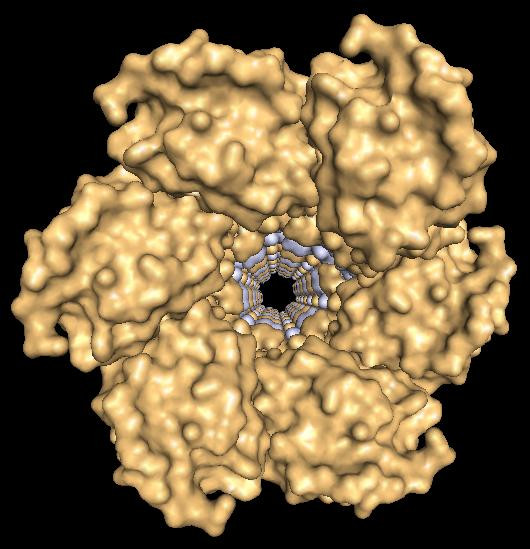
A new theory suggests that sperm may fertilise the egg by harpooning it. Some 14 years' worth of research from the University of Virginia School of Medicine has led scientists to the discovery of a protein – sperm lysozyme-like protein 1 or SLLP1 – that may latch the sperm onto the egg.
The research paper, which was published in Andrology, says that it is a big leap in helping scientists to better understand the structure of the sperm's acrosome – an organelle in the sperm head – and opens up a new theory regarding the process of fertilisation.
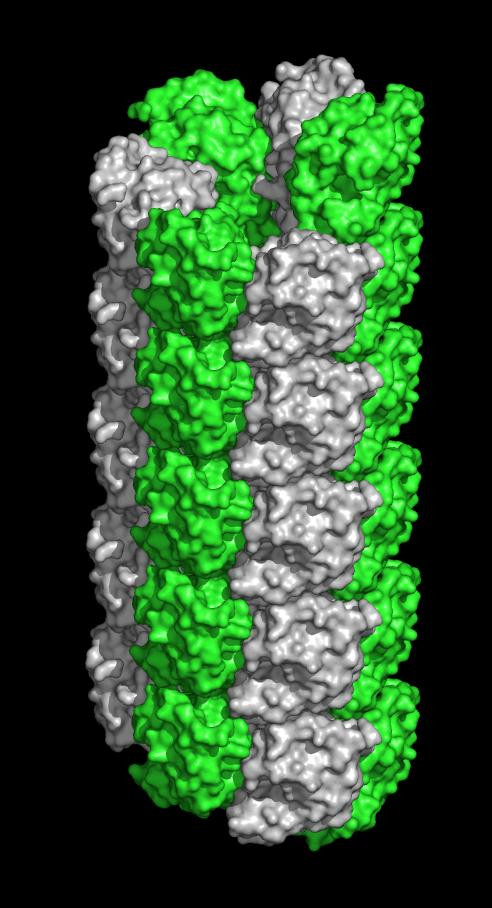
UVA reproduction researcher John Herr PhD, of the Department of Cell Biology, said: "This finding has really captured our imagination. One of the major proteins that is abundant in the acrosome [in the arterior region of the sperm head] is crystallizing into filaments, and we now postulate they're involved in penetrating the egg – that's the new hypothesis emerging from the finding, which leads to a whole new set of questions and new hypotheses about the very fine structure of molecular events during fertilization."
"This is an important protein, because it's the first crystal structure from a protein within the sperm acrosome," said Heping Zheng, the lead author of the paper outlining the discovery. "It is also the first structure of a mammalian sperm protein with a specific oocyte-side binding partner characterized. To our knowledge, only nine proteins specifically obtained from mammalian sperm have known structures."
The discovery comes after a collaboration between Herr's lab and the laboratory of Wladek Minor from the Department of Molecular Physiology and Biological Physics. It was the former's lab which discovered the protein but had no way to determine the shape and structure of it.
Minor's team however had the equipment to capture the protein within a static crystal, freeze it to prevent decay and shoot x-rays at it. By analysing how the x-rays were deflected, they could paint a picture of the protein.
"At the very fundamental level, understanding that fine molecular architecture leads me, the biologist, to be able to posit new functions for this family of proteins my lab discovered in the acrosome," Herr explained.
© Copyright IBTimes 2025. All rights reserved.





















