Japanese Researchers Invent a New Safer Lithium Battery That Uses Parasitic Conduction

Researchers at the Tohoku University in Japan have created a new type of lithium ion conductor that could greatly decrease the fire risks associated with lithium batteries.
Due to its long life, Lithium ion is currently the preferred choice of rechargeable battery for everything from air planes and electrical cars to wearable tech, smartphones, tablets and implantable medical devices.
However, the compound relies on liquid chemistries, whereby lithium salts dissolve into organic liquid electrolyte solvents that are highly flammable, so in order to remove fire risks, the cells in batteries would need to be completely solid-state.
To achieve this, the researchers used rock salt Lithium Borohydride (LiBH4). This compound has previously been considered for use in lithium batteries, but has so far only been able to work if it is at a high temperature or pressure.
Their research, entitled "Synthesis of rock-salt type lithium borohydride and its peculiar Li+ ion conduction properties", is published in the open access journal APL Materials.
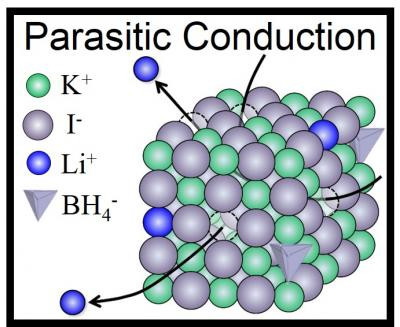
By applying LiBH4 to a cubic lattice of KI molecules (a process called "doping"), the researchers were able to stabilise the high-pressure form of Lithium Borohydride, which formed a solid solution at normal atmospheric pressure that was also stable at room temperature.
Usually, doping is performed by adding a small amount of stabilising element to an ionic conductor that is abundant in Lithium. However, in this case, the Li+ ions acted as if they were pure Li+ ion conductors, even though they were only meant to be doping the KI lattices.
"In other words, LiBH4 is a sort of 'parasite' but not a host material," said Hitoshi Takamura, who led the research at Tohoku University.
The researchers are calling this behaviour "parasitic conduction" and believe that this method could be used for other new batteries. For example, small amounts of Li+ ions could be used to dope an oxide, sulphide, halide or nitride host material.
"This work suggests the potential of this mechanism in the ongoing search for the perfect material for use in solid state batteries. The urgency of this quest has been abundantly clear after the grounding of so many aircraft in recent months," said Takamura.
© Copyright IBTimes 2025. All rights reserved.