Antidepressants and blood thinners cause brain cancer cells to eat themselves

A combination of antidepressants and blood thinners has been found to cause brain cancer cells to eat themselves. Scientists found lifespan doubled in a study on mice when treated with the two drugs for glioblastoma – the most common and aggressive malignant brain tumour.
A team of researchers at the Swiss Federal Institute of Technology (EPFL) were examining a connection between tricyclic antidepressants and brain cancer – a link that has been known about for around 15 years. Although there has been some evidence these drugs can lower the risk of developing aggressive glioblastomas, it showed no effect when trialled as a treatment.
However, when used in combination with blood thinners (known to increase the destruction of cells), researchers discovered they increased tumour autophagy, where cancer cells eat themselves. Their findings were published in the Cell Press journal Cancer Cell.
Mice were given the combination therapy for five days a week, with 10 to 15-minute intervals between the drugs. Antidepressants were given orally while the blood thinner was injected. Scientists believe the drugs work by disrupting the biological pathway that controls the rate of autophagy, working together to simulate the process and causing the cancer cells to die.
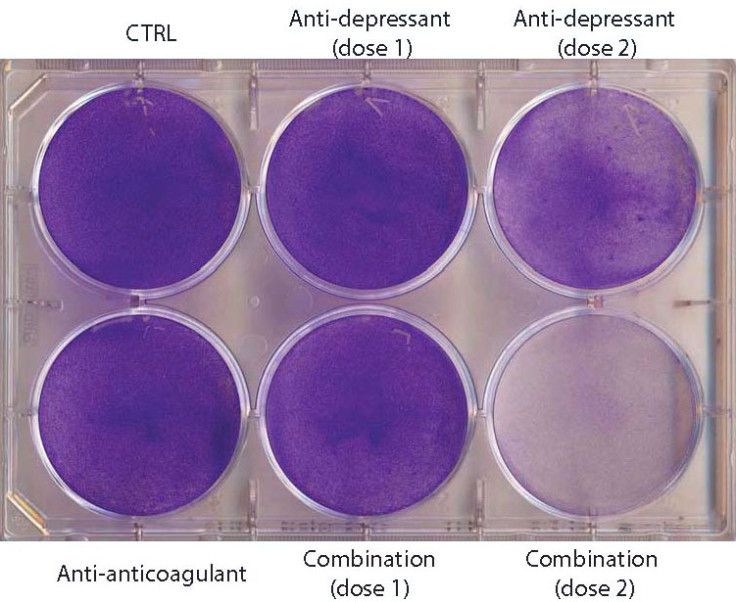
Both of the drugs used are already approved by the FDA and researchers believe the findings could translate to human models. "The results presented herein offer a provocative strategy to target apoptosis-resistant brain tumours by hyperactivating levels of cellular autophagy; such dual targeting of autophagy with repurposed, pharmacologically tractable drugs may warrant consideration for clinical trials," they wrote.
Senior study author Douglas Hanahan said: "It is exciting to envision that combining two relatively inexpensive and non-toxic classes of generic drugs holds promise to make a difference in the treatment of patients with lethal brain cancer. However, it is presently unclear whether patients might benefit from this treatment. This new mechanism-based strategy to therapeutically target glioblastoma is provocative, but at an early stage of evaluation, and will require considerable follow-up to assess its potential."
However, he was keen to point out the therapy did not cure the mice of the disease. Instead it delayed progression and extended lifespan. "It seems likely that these drugs will need to be combined with other classes of anticancer drugs to have benefit in treating gliblastoma patients," he said. "One can also envision 'co-clinical trials' wherein experimental therapeutic trials in the mouse models of glioblastom are linked to analogous small proof-of-concept trials in GBM patients. Such trials may not be far off."
© Copyright IBTimes 2025. All rights reserved.






















