France drug trial: Evidence of deadly dog tests before fatal brain damage dose to human subject

An investigation carried out by Le Figaro newspaper in France has gathered evidence that the molecule used in the Rennes clinical trial, which caused the death of one man in January 2016, may have caused the deaths of dogs in a preclinical testing phase. Bial, the pharmaceutical lab who ordered the trial, will not publish details of preclinical tests citing industrial secrecy issues.
Reacting to the news, one expert has said that the suspect deaths of animals, as well as the fact that very large doses were given to five participants on January 11, just a day after another was carried to hospital, show a lack of safety during the trial.
Danielle Piomelli, professor of neurobiology and pharmacology at the University of California told Le Figaro: "The fact that dogs died during the first phase of the trial is a crucial information. It is a real sign of alert".
However, Biotrial's president Francois Peaucelle told French channel BFMTV that despite the dogs'death, "the conclusions of this study were sufficiently clear and clean to rule out any particular ambiguity about proceeding with human tests."
French state prosecution has opened an investigation on how the trial was carried out by Biotrial, the company who implemented tests for Bial. A preliminary report by IGAS (National Inspection Comittee on Social Affairs), published on the 4th of February, absolved Biotrial, Bial and the French drug safety agency Agence Nationale de Sécurité du Médicament (ANSM) which approved the trial, of wrongdoing.
The BIA 10-2474 molecule tested by Bial was meant to target the central nervous system and help patients suffering from anxiety, mood swings as well as neurodegenerative disorders.
Brain damages and opacity
Meanwhile in Rennes, the doctors looking after the five persons who remain hospitalised have warned that they could suffer from potentially irreversible brain damages.
"Four patients out of the surviving five have neurological problems. Three have symptoms so severe that it leads us to worry that they will be disabled for the rest of their life. However, this prognosis is still not definitive" said Gilles Edan, who leads the neuroscience department at Rennes hospital.
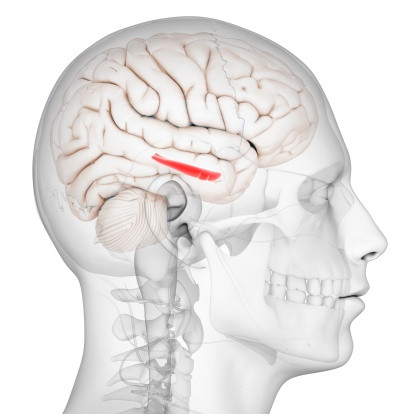
So far, more information about the extent of the brain damages have not been made public. Le Figaro says French sanitary authority ANSM, the institution responsible for approving clinical trials, is to blame.
It points out its opacity, and explains that its president, Dominique Martin, so far refuses to divulge information about how the molecule has impacted the brain of the trial's participants. "I can only say that the molecule has targeted an area at the base of the brain but the rest is a medical secret," he told French journalists.
According to Le Figaro, the hippocampus could be one of the areas most affected by the drug, leading to long term memory losses and spatial recognition problems.
Next month, an article will be published in the British Journal of Pharmacology to identify all the transparency issues that remain, and the mistakes made by ANSM, Biotrial, and Bial.
© Copyright IBTimes 2025. All rights reserved.






















