Mysterious molecular mechanism of key enzyme found in most life forms discovered
Scientists are a step closer to understanding an ancient, evolutionary enzyme that is key to maintaining healthy cells and has been linked to diseases such as diabetes and cancer.
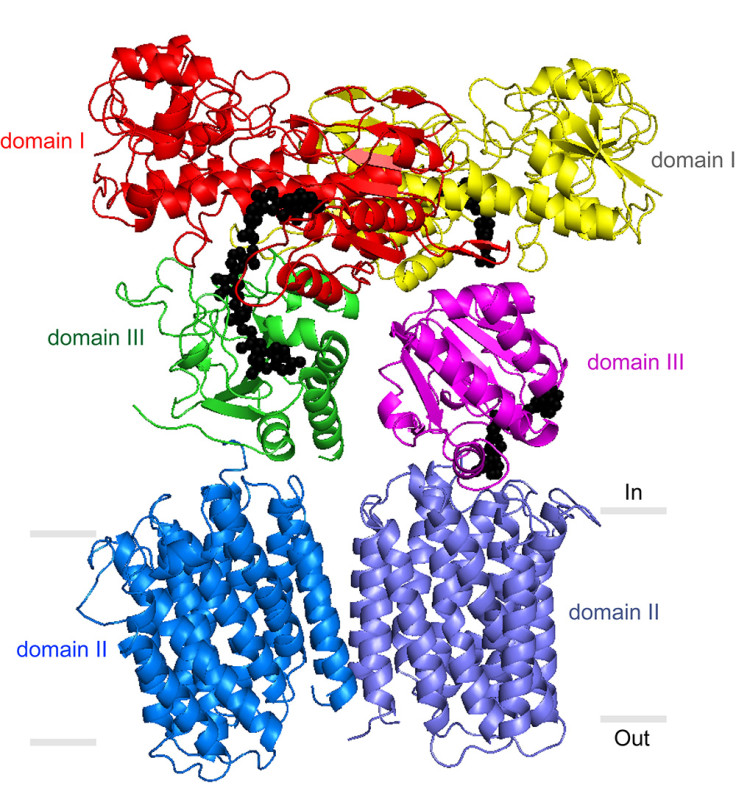
Biologists at The Scripps Research Institute (TSRI) were able to form crystals and determine the structure of TH, a metabolic enzyme produced in most forms of life on Earth.
The enzyme nicotinamide nucleotide transhydrogenase (TH) is found throughout the animal kingdom as well as in plants and many simpler species.
In humans and other higher organisms, it works within the mitochondria, the energy powerhouses of the cells. The enzyme works to regulate a proton transit process leading to the production of a compound NADPH that defuses free radicals and keep cells healthy.
Given its loose structure, it has been difficult to pin down the enzyme in full and how it assembles from the various parts existing across the membrane.
The team was able for the first time to form crystals of the TH transmembrane portion and use X-ray
crystallography to determine its structure to a high atomic resolution.
The team also was able to grow crystals of the whole TH enzyme.
The electron microscopy data confirmed that TH naturally exists as a two identical copies bound together.
These side-by-side structures are highly flexible and always have different orientations.
"Our most striking finding was that the two domain III structures are not symmetric—one of them faces up while the other faces down," said Josephine H Leung, a graduate student in the Stout laboratory who was lead author of the study.
One of the structures is oriented to catalyse the production of NADPH, while the other turned to the membrane facilitates transit of protons (charged hydrogen atoms) back into the mitichondria, the researchers surmise.
The structural model also suggests that with each proton transit, the two structures flip and switch their functions.
While more work remains to be done to determine TH's precise structure and mechanism, the findings are a big step toward understanding the intricate molecular mechanism of the enzyme.
The work has been published in Science.
© Copyright IBTimes 2025. All rights reserved.





















