Ozone-destroying chemicals not controlled by Montreal Protocol 'increasing rapidly'
Ozone-destroying chemicals that are not controlled by the United Nations are increasingly rapidly and are threatening to offset the gains made by the Montreal Protocol over 25 years ago.
Published in the journal Nature Geoscience, scientists discovered that one of the man-made 'very short-lived substances' (VSLS) in particular is growing at a very fast rate.
VSLS can be produced from both natural and industrial sources. However, the study authors noticed a rapid increase in dichloromethane, a VSLS used in a range of industrial processes.
Lead author Ryan Hossaini, from the University of Leeds, said: "Industrial production of VSLS is not controlled by the United Nations Montreal Protocol because historically these chemicals have contributed little to ozone depletion.
"But we have identified now that one of these chemicals is increasing rapidly and, if this increase is allowed to continue, it could offset some of the benefits to the Ozone Layer provided by the Montreal Protocol."
Researchers used a 3D computer model to work out the impact of VSLS on ozone and climate. They used measurements of these chemicals taken from the last 20 years, including those from the National Oceanic and Atmospheric Administration.
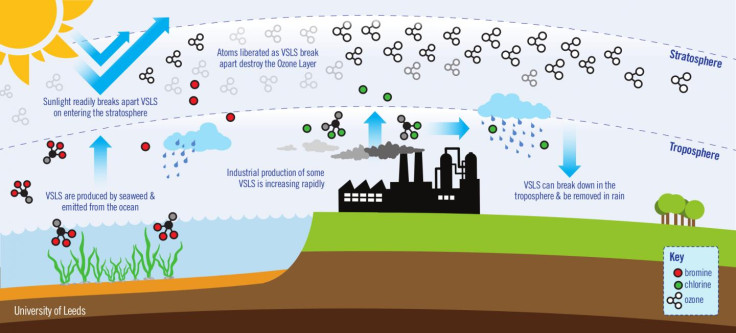
The authors found that although ozone depletion from VSLS is small compared to those covered by the Montreal Protocol, they were four times more efficient at influencing climate.
Hossaini said: "Due to their short atmospheric lifetimes, VSLS break down and destroy ozone in the lowermost part of the stratosphere. This is important, as a molecule of ozone lost in this region has a far larger impact on climate than a molecule destroyed at higher altitudes by longer-lived gases."
Findings also showed that 90% of ozone loss from VSLS is from naturally emitted sources, but that man-made VSLS contribution is increasing and will continue to do so over the coming years.
Co-author Dr Stephen Montzka, from the National Oceanic and Atmospheric Administration (NOAA), said: "The increases observed for dichloromethane are striking and unexpected; concentrations had been decreasing slowly in the late 1990s, but since then have increased by about a factor of two at sites throughout the globe."
Hossaini added: "It is uncertain what is driving this growth. However, it could be partly due to the fact that dichloromethane is used in the manufacturing process of some HFCs, the 'ozone-friendly' gases which were developed to replace CFCs. This would mean, ironically, that production of ozone-friendly chemicals is actually releasing some ozone-destroying gases into the atmosphere."
© Copyright IBTimes 2025. All rights reserved.






















