Physicists just created an exotic new state of matter by stuffing an atom full of atoms
The new research is the first proof of the new state of matter, called Rydberg polarons.
Scientists have just provided the first proof of the existence of an exotic new state of matter called Rydberg polarons. Physicists essentially created this state of matter by stuffing an atom full of more ordinary atoms.
The new state of matter is formed in highly cold temperatures, when an electron orbits the nucleus at a great distance, leaving enough space for other atoms to end up inside. These atoms combined, form a weak bond, creating Rydberg polarons.
To create the new state of matter, a team of American and Austrian scientists combined two different fields of atomic physics - Bose-Einstein condensates and Rydberg atoms. The researchers first created a Bose-Einstein condensate with strontium atoms. One of these atoms was then converted to a Rydberg atom with a massive atomic radius by transferring energy, using lasers.
So how far does an electron have to be from the nucleus to create a Rydberg atom?
"The average distance between the electron and its nucleus can be as large as several hundred nanometres - that is more than a thousand times the radius of a hydrogen atom", theoretical physicist Joachim Burgdörfer from TU Wien in Vienna, said in a statement.
The researchers found that the distance between the atoms within the condensate is much smaller than the distance between the electron and the nucleus. According to researchers, as many as 170 more strontium atoms could be ensconced in the massive orbit, depending on the Rydberg atom's radius and the density of the Bose-Einstein condensate.
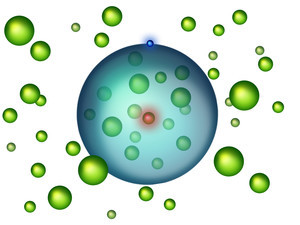
The researchers also discovered that these unusually grouped atoms have little to no impact on the Rydberg electron's orbital path. "The atoms do not carry any electric charge, therefore they only exert a minimal force on the electron", said Shuhei Yoshida, a physicist also from TU Wien.
However, the electron is still able to feel the presence of the neutral strontium atoms and is slightly scattered, though not enough to ever derail from its orbit. This kind of scattering is can be explained by the quantum physics of slow electrons, which also ensures that the electron is not transferred into a different state. This weak interaction between the strontium atoms and the orbiting electron reduces the total amount of energy in the system.
"It is a highly unusual situation", Yoshida said. "Normally, we are dealing with charged nuclei, binding electrons around them. Here, we have an electron, binding neutral atoms."
"For us, this new, weakly bound state of matter is an exciting new possibility of investigating the physics of ultracold atoms", added Burgdörfer. "That way one can probe the properties of a Bose-Einstein condensate on very small scales with very high precision."
The new research was published in the journal Physical Review Letters.






















