Created 'by accident' - New bendable smartphone battery has one-minute charge time
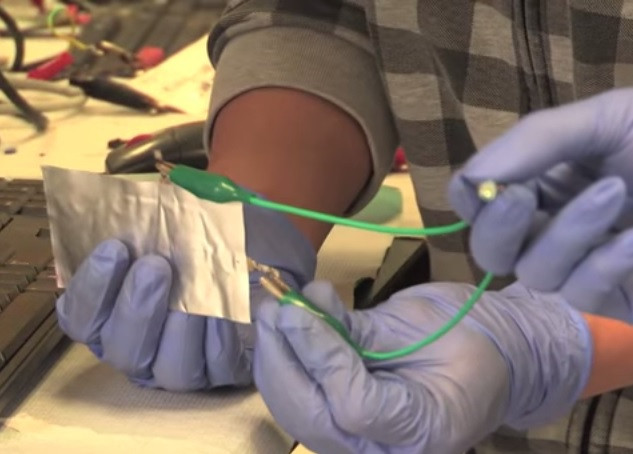
Researchers at Stanford University may have finally answered our smartphone wishes, by creating a battery which fully charges in just one minute - and is much safer than the lithium-ion technology we currently all use.
Called an aluminium power cell, the prototype battery could allow a smartphone to be charged in one minute, compared to the several hours most handsets currently take, and is so chemically stable it can be drilled through without catching fire. It is also claimed the battery can survive up to 7,500 charge cycles, seven times than of current lithium-ion batteries.
Hongjie Dai, professor of chemistry at Stanford University, California, said: "We have developed a rechargeable aluminium battery that may replace existing storage devices, such as alkaline batteries, which are bad for the environment, and lithium-ion batteries, which occasionally burst into flames. Our new battery won't catch fire, even if you drill through it. Lithium batteries can go off in an unpredictable manner - in the air, the car or in your pocket...I see this as a new battery in its early days. It's quite exciting."
An aluminum-ion battery consists of two electrodes: a negatively charged anode made of aluminum and a positively charged cathode.
Discovered by accident
"People have tried different kinds of materials for the cathode," Dai said. "We accidentally discovered that a simple solution is to use graphite, which is basically carbon. In our study, we identified a few types of graphite material that give us very good performance."
Such aluminium batteries have previously existed, but they suffered from poor longevity and would die after just 100 charge cycles, where the battery is fully drained then recharged back to 100%. But now 7,500 charge cycles can be achieved before the battery's performance suffers. Given most smartphones are charged nightly this would give them a life of 20 years.
Writing in the April edition of the Nature journal, researchers behind the battery wrote: "This was the first time an ultra-fast aluminium-ion battery was constructed with stability over thousands of cycles."
Co-lead author of the report, Ming Gong, said the battery's stability means it is flexible. "You can bend it and fold it, so it has the potential for use in flexible electronic devices. Aluminium is also a cheaper metal than lithium."
© Copyright IBTimes 2025. All rights reserved.






















